Case Report - (2022) Volume 9, Issue 4
Cavernous hemangioma of the spermatic cord in children: A case report
Cedric Bignon Ulrich Assouto1, Codjo Serge Metchihoungbe2, Amoussou Sedjro Clotaire Romeo Houegban1* and Beaudelaire Romulus Assan1Abstract
Hemangioma is the most benign common vascular tumor of childhood. They are classified as capillary, cavernous, arteriovenous, venous and mixed. The cavernous and mixed types are the most common. Cavernous hemangiomas can occur at different sites in the human body. The most common sites are the musculoskeletal system, the liver and the spleen and more rarely the genitals. The spermatic cord is an exceptional site. We present a case of cavernous hemangioma of the spermatic cord in an 11-month-old-male infant and review the related literature. He underwent a monobloc surgical resection. The postoperative course was simple. The infant discharged from hospital the next day. At a 1-year follow-up, there was no recurrence. Cavernous hemangioma of the spermatic cord is a very rarely diagnosed tumor in infants. It is most often discovered with another associated pathology. It must be suspected in front of a painless scrotal mass. The course is usually uneventful after surgery.
Keywords
Cavernous hemangioma, spermatic cord, exceptional, infant
Introduction
Hemangioma is the most common vascular tumor of childhood. It affects 1-3% of new-borns and 10% of children by the age of one year [1]. Hemangiomas are benign vascular tumors. They are classified as capillary, cavernous, arteriovenous, venous and mixed [2]. The cavernous and mixed types are the most common. Cavernous hemangiomas can occur at different sites in the human body. The most common sites are the musculoskeletal system, the liver and the spleen [3]. About 2% of hemangiomas involve the genitals [4]. The spermatic cord is an exceptional site.
Clinical signs are non-specific, making clinical diagnosis difficult. Ultrasound and magnetic resonance imaging can be of great help in thinking about the diagnosis. Treatment often consists of surgical excision, but other therapeutic alternatives exist. Recurrence is possible [1,4]. We hereby present a case of cavernous hemangioma of the spermatic cord in an infant and review the related literature.
Case Presentation
An 11-month-old male child, without particular past medical history, waiting for a cure for a left inguinoscrotal hernia, was referred to our department for a strangulation episode. Physical examination revealed a painful, soft and unimpulsive left inguinoscrotal swelling, with negative trans illumination test. The right scrotum was normal. After reduction of swelling under diazepam, a small, solid, rounded mass was palpated above the left testis with a negative trans illumination test.
A testicular ultrasound showed a mixed structure, located above the left testis. Magnetic resonance imaging of the scrotum and CT of the scrotum were not available. We decided to proceed with surgical exploration using a left inguinal approach under general anaesthesia.
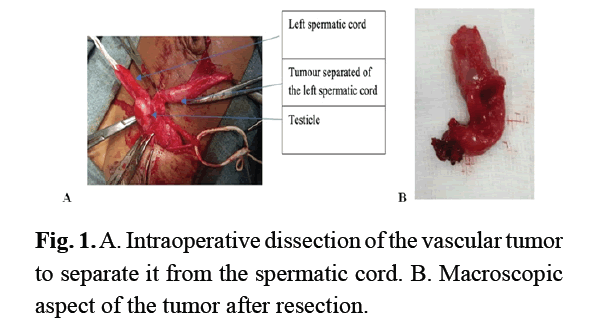
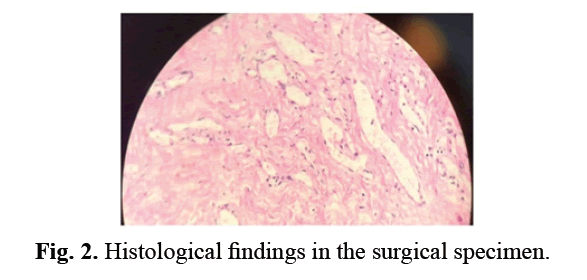
A persistence of the left peritoneal-vaginal canal was identified, which we ligated to the deep inguinal orifice. Further exploration revealed a solid, oval mass developed at the expense of the vaginal tunica, measuring about 10 mm in length (Fig 1A). This mass was dissected and then resected in monobloc form (Fig 1A). During this dissection, the ascending of the testis led to left orchidopexy. The postoperative course was simple. The infant discharged from hospital the next day. The child was reviewed at 1 month, 3 months, and 1 year postoperatively. At a 1-year follow-up, there was no recurrence. Histological examination of the surgical specimen showed a tumor formation composed of large vascular cavities covered with a regular and turgescent endothelium filled with red blood cells (Fig 2). It is associated with fibrous bands. It was therefore concluded to be a cavernous hemangioma.
Figure 1: A. Intraoperative dissection of the vascular tumor to separate it from the spermatic cord. B. Macroscopic aspect of the tumor after resection.
Figure 2: Histological findings in the surgical specimen.
Results and Discussion
The International Society for the Study of Vascular Anomalies (ISSVA) has classified vascular abnormalities into two groups: vascular tumors and vascular malformations. Vascular tumors are primarily due to endothelial hyperplasia and may regress spontaneously. Vascular malformations occur as localized defects of vascular morphogenesis, secondary to dysfunction of the regulatory pathways of embryogenesis and generally persist into adulthood [5]. The natural history of a skin or soft tissue lesion, such as a hemangioma that appears shortly after birth, involves several stages. First, it rapidly increases in size over the months and then decreases in size over the years. This means that at birth, there may be no sign of hemangioma. This is the case of our patient for whom the swelling was discovered incidentally at 11 months of age when it was properly monitored, showing its progressive increase in volume. However, depending on the location, 30% of children will have a “mark” (red mark), a discolored or localized mark (telangiectasia) surrounded by a pale halo at the site of the future hemangioma [6]. The tumor is initially solid with few recognizable vascular structures within it. It may be rich in mitosis-abundant cells with nuclear hyperchromia, which gives it a worrying histological appearance. Patients’ age is essential to differentiate a childhood hemangioma from an angiosarcoma because the latter is rare in children [6]. This cellular hyperactivity leads to the growth of the hemangioma until about 20 months of age. After this period of rapid expansion, the hemangioma stabilizes and grows at the same rate as the child. Hemangioma involution can occur up to 5 years [6].
At urogenital level, it can also be associated with acute spermatic cord torsion [7] or develop to the detriment of testicular parenchyma [6,8]. Other authors have described hemangiomas on the skin of the scrotum that can extend to the pelvic region and the penis [4,9,10]. On ultrasound, testicular hemangiomas are often hypoechoic but sometimes hyperechoic or even heterogeneous, as we found in our patient [7]. When tumor markers are negative, the presence of a mass with calcifications of variable size is strongly suggestive of a cavernous hemangioma [7]. However, serum levels of alpha-fetoprotein, beta-HCG and LDH increased in only half of the patients with malignant urogenital tumors. Differential diagnosis should therefore include germ cell tumors (seminoma, teratoma), adenomatoid and stromal sex cord tumors such as Sertoli cell tumor [7].
There are no clear guidelines for the treatment of hemangiomas in the urogenital sphere. Treatment options are numerous, ranging from oral corticosteroid therapy to surgery (excision of the tumor) and laser destruction of superficial blood vessels [10]. The indication depends on the topography of the tumor. In our patient, dissection and removal in one-piece was possible. Nevertheless, therapeutic abstention remains a valid option, especially if the diagnosis is straightforward. In our case, in the lack of additional investigations, surgical exploration for an excisional biopsy is justified. The evolution is good and no case of recurrence has been described in the literature after surgical removal as we have observed in our patient [7].
Conclusion
Our approach to intervention successfully identified and obliterated the accessory urethra without presenting any risk to the primary urethra, sphincteric mechanism, or neurovascular bundles. Our case illustrates a safe, familiar, and effective technique for localizing and excising an Effmann type IIA-1 urethral duplication while preserving the primary urethra, sphincteric mechanism, and neurovascular structures. As there is limited literature on the subject, we hope the contribution of our experience will provide guidance to other surgeons caring for children with this rare anomaly.
Cavernous hemangioma of the spermatic cord is a very rarely diagnosed tumor in infants. It is most often discovered with another associated pathology. It must be suspected in front of a painless scrotal mass. The course is usually uneventful after a monobloc surgical resection.
References
- Dinehart SM, Kincannon J, Geronemus R. Hemangiomas: Evaluation and treatment. Dermatol Surg. 2001; 27(5):475-485.
[Cross Ref] [Google Scholar] (All version) [Pubmed]
- Ergün O, Ceylan BG, Armagan A, et al. A giant scrotal cavernous hemangioma extending to the penis and perineum: A case report. Kaohsiung J Med Sci. 2009; 25(10):559-561.
[Cross Ref] [Google Scholar] (All version) [Pubmed]
- Ojili V, Tirumani S, Gunabushanam G, et al. Abdominal Hemangiomas: A Pictorial review of unusual, atypical, and rare types. Can Assoc Radiol J. 2013; 64:18-27.
[Cross Ref] [Google Scholar] (All version) [Pubmed]
- Ferrer FA, McKenna PH. Cavernous hemangioma of the scrotum: A rare benign genital tumor of childhood. J Urol. 1995; 153(4):1262-1264.
[Cross Ref] [Google Scholar] (All version) [Pubmed]
- Talmon GA, Stanley SM, Lager DJ. Capillary hemangioma of the testis. Int J Surg Pathol. 2011; 19:398–400.
[Cross Ref] [Google Scholar] (All version) [Pubmed]
- Kolligian ME, Kogan SJ, Beneck D. Intrascrotal Hemangioendothelioma In Infancy. Urology. 1997; 50:456-458.
[Cross Ref] [Google Scholar] (All version) [Pubmed]
- Tepeneu NF, Krafka K, Meglic S, et al. Testicular cavernous hemangioma associated with testicular torsion: Case report and review of literature. Int. J. Surg. Case Rep. 2018; 49:247-250.
[Cross Ref] [Google Scholar] (All version) [Pubmed]
- Fossum BD, Woods JC, Blight EM Jr. Cavernous hemangioma of testis causing acute testicular infarction. Urology. 1981; 18(3):277-278.
[Cross Ref] [Google Scholar] (All version) [Pubmed]
- Elbaghouli M, Aboutaib R, Dakir M, et al. Cavernous hemangioma of the spermatic cord: About a case and literature review. Basic Clin Androl. 2010; 20 (2):155-158.
[Google Scholar] [Pubmed]
- Alp BF, Kopru B, Topuz B, et al. Case report: Urogenital hemagioma (UGH). Eur Urol Suppl. 2015; 14(8):137-139.
Author Info
Cedric Bignon Ulrich Assouto1, Codjo Serge Metchihoungbe2, Amoussou Sedjro Clotaire Romeo Houegban1* and Beaudelaire Romulus Assan12Department of Pediatric Surgery, National University Hospital Center “Hubert Koutoukou Maga”, University of Abomey- Calavi, Cotonou, Benin
Received: 29-Aug-2022, Manuscript No. PUCR-22-73128; , Pre QC No. PUCR-22-73128; Editor assigned: 31-Aug-2022, Pre QC No. PUCR-22-73128; Reviewed: 12-Sep-2022, QC No. PUCR-22-73128; Revised: 22-Sep-2022, Manuscript No. PUCR-22-73128 (R); Published: 29-Sep-2022, DOI: 10.14534/j-pucr.20222675585
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.