Case Report - (2022) Volume 9, Issue 3
Irregular bladder smooth muscle actin-gamma 2 expression in ACTG2 mutation-associated Megacystis Microcolon Intestinal Hypoperistalsis Syndrome (MMIHS): A case report
Peter Heinz-Erian1*, Elisabeth Bruder2, Andreas Janecke1, Peter Rehder3, Heinz Zoller4, Bettina Zelger5, Markus Pirklbauer6 and Thomas Müller6Abstract
The majority of published cases of Megacystis Microcolon Intestinal Hypoperistalsis Syndrome (MMIHS), a disorder of smooth muscle function of hollow visceral organs is associated with mutations in the actin gamma2 (ACTG2) gene on chromosome 2p13. While alterations in smooth muscle actin histology have been described for the gastrointestinal tract no such information is available for urogenital organs of patients with ACTG2 mutation-based MMIHS. We report the first description of actin gamma2 pathohistology in bladder smooth muscle of a male MMIHS-patient bearing a de novo ACTG2-mutation (c.770G>A; p.R257H) who suffered from voiding insufficiency due to severely deficient bladder contractility as demonstrated by video-urodynamic investigations. Our findings are a further contribution to the elucidation of pathogenic mechanisms of functional myopathic bladder atony.
Keywords
ACTG2-mutation, functional bladder atony, MMIHS, urology, myopathy
Introduction
Megacystis Microcolon Intestinal Hypoperistalsis Syndrome (MMIHS) is a myopathic disorder caused by a variety of genetic defects affecting the smooth muscle cell contraction apparatus of both the urogenital and gastrointestinal tracts [1]. Clinically, MMIHS is characterized by megacystis (bladder distension in the absence of mechanical obstruction), microcolon and intestinal hypoperistalsis [1,2]. Presently, the most thoroughly characterized underlying pathogenetic mechanism of MMIHS is that of actin gamma2 dysfunction which has been associated with mutations in the actin gamma 2 (ACTG2) gene [3]. For the gastrointestinal tract, reports on patients with MMIHS provide pathohistological information on granular abnormalities in actin gamma 2 filament formation, subcellular localization and organization in smooth muscle cells as well as on functional consequences in terms of smooth muscle contraction [4,5].
While smooth muscle actin alterations have been published in the urinary tract of patients with MMIHS of unknown genetic origin [6], no smooth muscle histology of the bladder has been reported for ACTG2 mutationassociated MMIHS to date. We describe for the first time histopathological and immunohistochemical changes in smooth muscle actin gamma2 structure in the urinary tract of a patient suffering from MMIHS associated with a de novo heterozygous mutation (c.770G>A; p.R257H) in the ACTG2 gene.
Case Presentation
The male patient was born at term to a couple of Serbian caucasian origin who were fourth degree cousins. During his first years of life he suffered from predominantly gastrointestinal problems including intractable constipation which led to repeated partial resections of the ileum and colon eventually resulting in subtotal ileo-colectomy. Hirschsprung´s disease was histologically excluded. During the following years the patient was frequently hospitalized because of repeated episodes of electrolyte and acid-base imbalance following intermittent bouts of severe diarrhea. After multiple complications including adhesion ileus causing further laparotomies, progressive intestinal failure and several urinary tract infections, the 17-year old adolescent was admitted to our hospital for further diagnostic evaluation.
He presented with severe dehydration, cachexia (weight 36 kg, height 157 cm), bloated abdomen, watery diarrhea and hypochloremic alkalosis. Congenital Chloride Diarrhea (CCD) was suspected, however, mutational analysis of the SLC26A3 gene was normal, excluding this disorder. During the subsequent six months the patient was successfully weaned from parenteral nutrition and was able to maintain near normal oral alimentation with frequent meals (eight to ten times daily) and substitution of large amounts of oral fluid, NaCl (upto 32 mmol/kg/d) and KCl (upto 20 mmol/kg/d). Two years later the patient´s weight was 54 kg, his height was 173 cm. Under this regimen he was able to maintain rather good health, to successfully conclude his college graduation exams and obtain his driver´s license. He even managed to drive his car on his own for more than 1000 km from his hometown to our hospital for medical control visits.
From the age of 23 years on he suffered from increasingly frequent bouts of urinary tract infections. Increased bladder volume, and a completely atonic bladder with no increase in detrusor pressure were diagnosed by ultrasound and video-urodynamic investigations (Fig 1). Radiographic images showed atonic bladder contours with unsatisfactory spontaneous emptying even on deliberate exertion of manual abdominal pressure. Transurethral resection of the prostate and bladder neck was performed. Light microscopy of bladder neck tissue showed atrophic smooth muscle cells surrounded by reticular fibrosis shown in Figs 2a and 2b as well as decreased and irregular granular smooth muscle actin expression by immunohistochemistry can be viewed in Fig 2c. There were also signs of chronic nonspecific inflammation but no discernible pathology of the neural network. Thinning and fibrosis of the smooth muscle in the internal bladder sphincter were similar to those found in full thickness biopsies from the patient´s rectum (not shown). Together with the findings of intestinal hypoperistalsis this suggested MMHIS, a hollow visceral myopathy, a diagnosis that was finally confirmed by the genetic finding of a de novo mutation in the ACTG2 gene (c.770G>A; p.R257H) on chromosome 2p13.1.
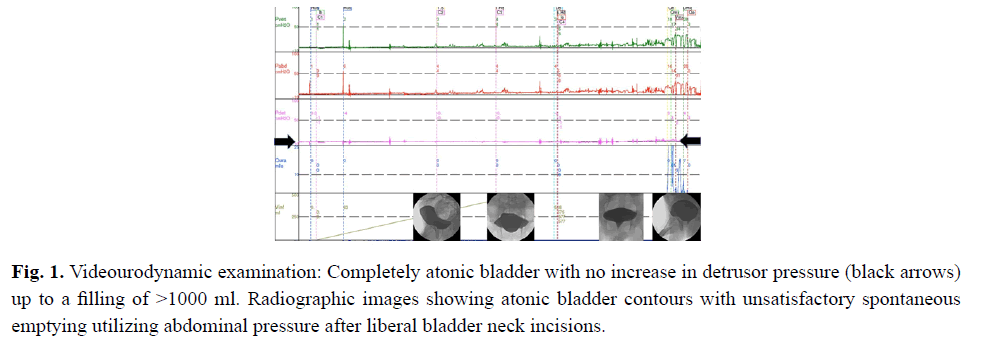
Figure 1: Videourodynamic examination: Completely atonic bladder with no increase in detrusor pressure (black arrows) up to a filling of >1000 ml. Radiographic images showing atonic bladder contours with unsatisfactory spontaneous emptying utilizing abdominal pressure after liberal bladder neck incisions.
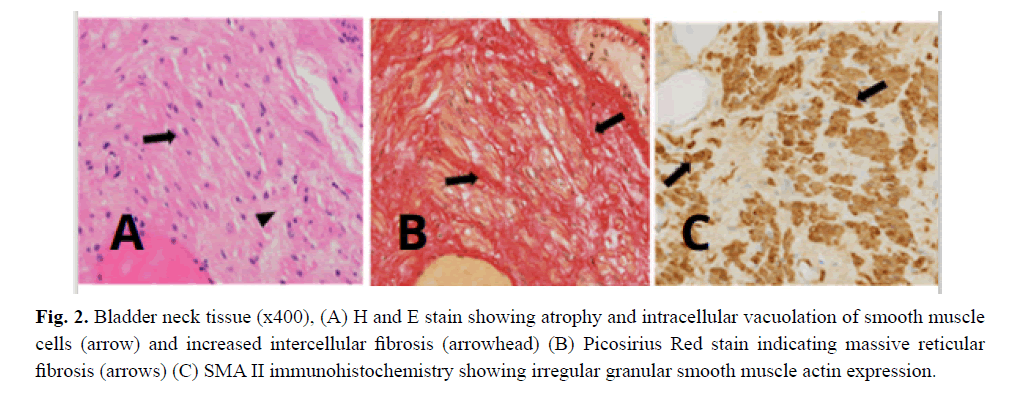
Figure 2: Bladder neck tissue (x400), (A) H and E stain showing atrophy and intracellular vacuolation of smooth muscle cells (arrow) and increased intercellular fibrosis (arrowhead) (B) Picosirius Red stain indicating massive reticular fibrosis (arrows) (C) SMA II immunohistochemistry showing irregular granular smooth muscle actin expression.
Further frequent urinary tract infections occurred even after a perineostoma to facilitate emptying of the bladder had been applied. Renal function gradually deteriorated and renal dialysis had to be initiated soon after his 28th birthday. The possibility of multivisceral transplantation including kidneys and intestine was considered but rejected because of widespread colonization with multiresistant bacteria. The patient died shortly after his 30th birthday in the course of another severe bout of urosepsis in his hometown hospital.
Results and Discussion
Megacystis and bladder atony are key features of MMIHS and are often associated with hydronephrosis, frequent urinary tract infections and renal insufficiency [7]. In our patient, apart from a few urinary tract infections which had been successfully treated without any problem on all occasions, no megacystis was diagnosed before the age of 22 years. Considering that the majority of MMIHS patients are diagnosed with megacystis and/or hydronephrosis prenatally or during the first year of life [8], our patient´s disease course was very unusual and cannot be explained by the available information. Even at the age of 22 years an ultrasound scan of the bladder wall was reported to be unremarkable although hydronephrosis had been diagnosed before. Two years later, however, a voiding cysto-urethrogram demonstrated a large-capacity bladder with one liter of residual volume of urine which did not improve after bladder neck dilatation and not even after bladder neck resection.
The latter procedure allowed us to obtain smooth muscle-containing tissue from the internal sphincter for microscopic evaluation which by demonstrating irregular smooth muscle actin expression shed further light on the probable mechanism of the patient´s defective bladder function. Unfortunately, we were unable to obtain bladder wall tissue for more specifically defining actin gamma2 pathohistology in the detrusor muscle, which harbours the main effector mechanism for bladder evacuation. Nevertheless, our observation of actin gamma2 deficiency in the internal bladder sphincter, the structure that normally constricts the internal orifice of the urethra but is non-functional in MMIHS, may provide an important clue regarding the patient´s voiding inefficiency. This latter mechanism may, besides stasis of urine in the bladder, also have been one factor in our patient´s propensity for frequent urinary tract infections caused by bacteria ascending from the penile orifice.
Thus far, to the best of our knowledge, smooth muscle actin gamma2 pathohistology in the atonic bladder of a patient with MMIHS associated with a de novo heterozygous mutation in the ACTG2 gene has not been reported and will thus provide further insight into the underlying mechanisms of myopathic bladder atony.
Conclusion
This course of our patient´s disease, besides typical early onset gastrointestinal symptomatology, was characterized by uro-nephrologic dysfunction that became manifest at an unusually late age. Our report on deficient actin pathohistology in the bladder provides additional information on the mechanism of disordered voiding in ACTG2-mutation-based MMIHS and is a further step in unraveling myopathic mechanisms of functional bladder atony.
Consent
Patient consent form has been obtained.
References
- Ambartsumyan L. Megacystis-microcolon-intestinal hypoperistalsis syndrome overview. GeneRev®. 2019.
[Google Scholar] [PubMed]
- Wangler MF, Beaudet AL. ACTG2-related disorders. 2015.
- Wangler MF, Gonzaga-Jauregui C, Gambin T, et al. Heterozygous de novo and inherited mutations in the smooth muscle actin (ACTG2) gene underlie megacystis-microcolon-intestinal hypoperistalsis syndrome. PLoS Gene. 2014;10(3):e1004258.
[Cross Ref] [Google Scholar] [PubMed]
- Hashmi SK, Barka V, Yang C, et al. Pseudo-obstruction–inducing ACTG2R257C alters actin organization and function. JCI Insight. 2020;5(16).
[Cross Ref] [Google Scholar] [PubMed]
- Halim D, Hofstra RM, Signorile L, et al. ACTG2 variants impair actin polymerization in sporadic megacystis microcolon intestinal hypoperistalsis syndrome. Hum Mol Genet. 2016;25(3):571-83.
[Cross Ref] [Google Scholar] [PubMed]
- Rolle U, Puri P. Structural basis of voiding dysfunction in megacystis microcolon intestinal hypoperistalsis syndrome. J Pediatr Urol. 2006;2(4):277-84.
[Cross Ref] [Google Scholar] [PubMed]
- Gosemann JH, Puri P. Megacystis microcolon intestinal hypoperistalsis syndrome: systematic review of outcome. Pediatr Surg Int. 2011;27(10):1041-6.
[Cross Ref] [Google Scholar] [PubMed]
- Hugar LA, Chaudhry R, Fuller TW, et al. Urologic phenotype and patterns of care in patients with megacystis microcolon intestinal hypoperistalsis syndrome presenting to a major pediatric transplantation center. Urology. 2018;119:127-32.
[Cross Ref] [Google Scholar] [PubMed]
Author Info
Peter Heinz-Erian1*, Elisabeth Bruder2, Andreas Janecke1, Peter Rehder3, Heinz Zoller4, Bettina Zelger5, Markus Pirklbauer6 and Thomas Müller62Department of Pathology, University Hospital Basel, Basel, Switzerland
3Department of Urology, Medical University of Innsbruck, Innsbruck, Austria
4Department of Medicine-I, Medical University of Innsbruck, Innsbruck, Austria
5Department of Pathology, Medical University of Innsbruck, Innsbruck, Austria
6Department of Medicine-IV, Medical University of Innsbruck, Innsbruck, Austria
Received: 07-Jun-2022, Manuscript No. PUCR-22- 66122; , Pre QC No. PUCR -22-66122 (PQ); Editor assigned: 10-Jun-2022, Pre QC No. PUCR -22-66122 (PQ); Reviewed: 27-Jun-2022, QC No. PUCR-22-66122; Revised: 04-Jul-2022, Manuscript No. PUCR-22-66122 (R); Published: 12-Jul-2022, DOI: 10.14534/j-pucr.2022267578
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
