Case Report - (2024) Volume 11, Issue 1
Simultaneous presentation of wilms tumor and obstructing duodenal-jejunal hematoma: A case report
Ethan Sandler1*, Helena Crowley2, Vijay Krishnan3, Hamad Aljutaili3, Maram Aljuaid3,4 and Regina Macatangay5Abstract
Herein, we discuss the unusual case of a patient with Wilms tumor and completely obstructing duodenal- jejunal hematoma of unclear etiology. A 5 year old female presented with one week of abdominal pain, non-bloody non-bilious emesis, and hematuria. On CT imaging, a right sided renal mass along with an obstructing duodenal and jejunal hematoma were identified. Upon surgical exploration, the tumor was biopsied, but not resected due to its proximity and dense adherence to the duodenum and other major vascular structures. A Gastrostomy Tube (GT) and a Jejunostomy Tube (JT) were placed to bypass the hematoma. Biopsies indicated Wilms Tumor (WT) with favorable histology. The subsequent treatment plan included nine cycles of chemotherapy with return to or for tumor excision after 7 weeks of chemotherapy. Repeat imaging about 6 weeks after initial presentation indicated interval increase in size of right renal mass but resolution of hemoperitoneum and duodenal hematoma. Following tumor excision, the patient received whole abdomen radiation due to tumor rupture through the capsule along with chemotherapy protocol per AREN-0533-DD-4A for 25 weeks. This case represents a previously undocumented associated intraluminal duodenal and jejunal hematoma and a path to safe tumor excision in the presence of challenging anatomic tumor adherence.
Keywords
Wilms tumor, Idiopathic duodenal hematoma
Introduction
Wilms Tumor represents about 5% of pediatric tumors, with about 500 cases per year reported in the United States. [1] The standard of care for unilateral WT is a complete surgical resection of the mass if possible with a radical nephrectomy. If the tumor is unresectable, then a biopsy of the mass to confirm a diagnosis of WT should be obtained with the plan to complete 6-9 weeks of chemotherapy with subsequent reassessment of resectability. The treatment team is multidisciplinary and includes pediatric radiologists, pediatric oncologists, pediatric surgeons, and radiation oncologists. It is not uncommon for the tumor to rupture through its capsule with a resultant peri-renal hematoma. There have been rare reports of similar presentations, with one report of a patient who died from duodenal hemorrhage after completing chemotherapy despite being in remission
[2]. It is rare that a WT directly invades the duodenum, with a resultant obstruction occurring [3], but it is not previously reported to have an associated obstructing duodenal hematoma without any evidence of direct invasion or vascular injury, as is present in this clinical scenario.
Case Presentation
A 5 year old African American female presented to an outside hospital with 5 days of abdominal pain, non- bloody, non-bilious emesis, and gross hematuria on the day of presentation. Upon arrival to the outside hospital, pelvic and abdominal ultrasound was obtained, showing a right renal mass. The patient was transferred to our Pediatric Emergency Department for further evaluation. She was previously a healthy 5 year-old girl with no significant past medical history or surgeries. Her mother had a history of multiple renal stones with subsequent failure of one of her kidneys requiring future nephrectomy. The patient took no daily medications and immunizations were up to date. She did not have any known allergies.
Clinical findings
On physical exam, vitals were indicative of hypertension with systolic BP up to 145 mm Hg and tachycardia with resting heart rate in 130s. She also had significant abdominal distention with moderate to severe tenderness to palpation, most prominent on the right side, with guarding. Deep palpation and rebound were not assessable due to severe pain and guarding. She had right Costovertebral angle tenderness (Table 1).
| Hospital Day 1 | Patient was admitted after ultrasound indicated right renal mas; CT Chest did not demonstrate metastatic disease. Amlodipine was started for hypertension with Hydralazine as needed. |
| Hospital Day 2 | Sedated MR Abdomen/Pelvis was indicative of a larger heterogeneous right renal mass, hematoma along the right kidney, hematoma extending from tumor across the midline concerning for intramural duodenal hematoma, and mild to moderate hemoperitoneum. |
| Hospital Day 3 | Patient underwent exploratory laparotomy with resection, however due to close adherence of mass to medial structures, complete resection was not performed. Tumor biopsies x2 were obtained. Frozen pathology indicated Wilms Tumor (WT) vs nephroblastoma. Nasogastric (NG) and G-tube (GT) were placed for decompression, and a J-Tube (JT) was placed for enteral feeds. Patient was admitted to PICU for post-operative care. |
| Hospital Day 7 | Medications and Pedialyte were started through the JT. |
| Hospital Day 11 | NG tube was removed. |
| Hospital Day 14 | Upper GI study obtained with contrast moving through the small bowel. Pathology results returned for WT with favorable histology. |
| Hospital Day 15 | Patient started chemotherapy with Dactinomycin and Vincristine per PED AREN 0533-DD-4A. |
| Hospital Day 64 | Patient returned to the Operating Room (OR) for exploratory laparotomy, lysis of adhesions, right radical nephrectomy, replacement of GT, and replacement of JT. A left Internal Jugular (IJ) Central Line was placed in the PICU post-operatively. |
| Hospital Day 71 | Patient returned to the or for placement of port for permanent access after having Peripherally Inserted Central Catheter (PICC), extended Peripheral IV (ePIV), and left IJ central line previously for access. She was discharged and followed as an outpatient for chemotherapy. Several admissions were scheduled for IV hydration prior to nephrotoxic chemotherapy. |
| Post-presentation Day 232 | Patient completed chemotherapy per protocol PED AREN 0533-DD-4A. There were multiple delays and missed appointments during chemotherapy course due to myelosuppression and family health needs. |
Table 1. A brief timeline of major events surrounding the patient’s hospitalization and care.
Diagnostic assessment
Initial workup at outside hospital demonstrated a moderate normocytic anemia (Hemoglobin 8.4 g/dL, MCV 75.8 fL) with elevated inflammatory markers (C-reactive protein 20.3 mg/dL) and evidence of hematuria without bacteriuria on urinalysis.
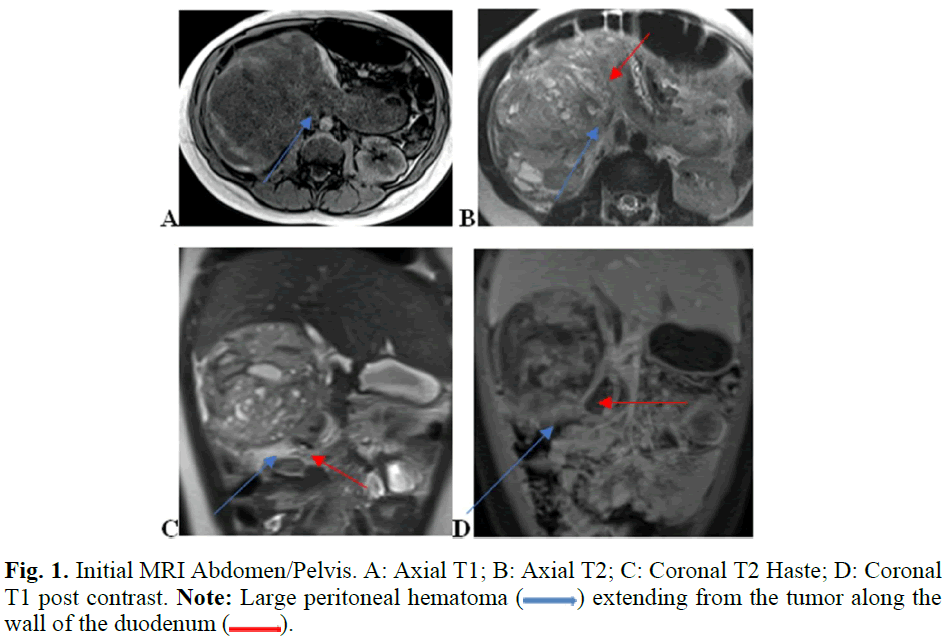
Pelvic ultrasound was obtained to further assess hematuria or vaginal bleeding which indicated free fluid in pelvis, but no masses were appreciated. Abdominal ultrasound indicated a right renal mass, prompting transfer to pediatric hospital. CT Chest without contrast was obtained which showed no evidence of mass in the chest. MR Abdomen with and without contrast was obtained preoperatively, which demonstrated 9.1 x 9.3 x 8.4 cm heterogenous and partially cystic exophytic soft tissue mass arising from the anterior aspect of the right kidney at the mid to lower pole region. There was also a large duodenal hematoma, and a large volume of hemoperitoneum was noted suggesting rupture of the tumor capsule. No metastases were identified. No vascular invasion was appreciated on MRI or US vascular studies. An Upper GI could not be performed as the patient did not tolerate oral contrast (Figures 1 and 2).
Fig. 1. Initial MRI Abdomen/Pelvis. A: Axial T1; B: Axial T2; C: Coronal T2 Haste; D: Coronal T1 post contrast. Note: Large peritoneal hematoma ( ) extending from the tumor along the wall of the duodenum (
) extending from the tumor along the wall of the duodenum ( ).
).
Fig. 2. Initial MRI Sagittal T2 weighted image demonstrating the large renal mass ( ) with resultant upper collecting system obstruction (
) with resultant upper collecting system obstruction ( ).
).
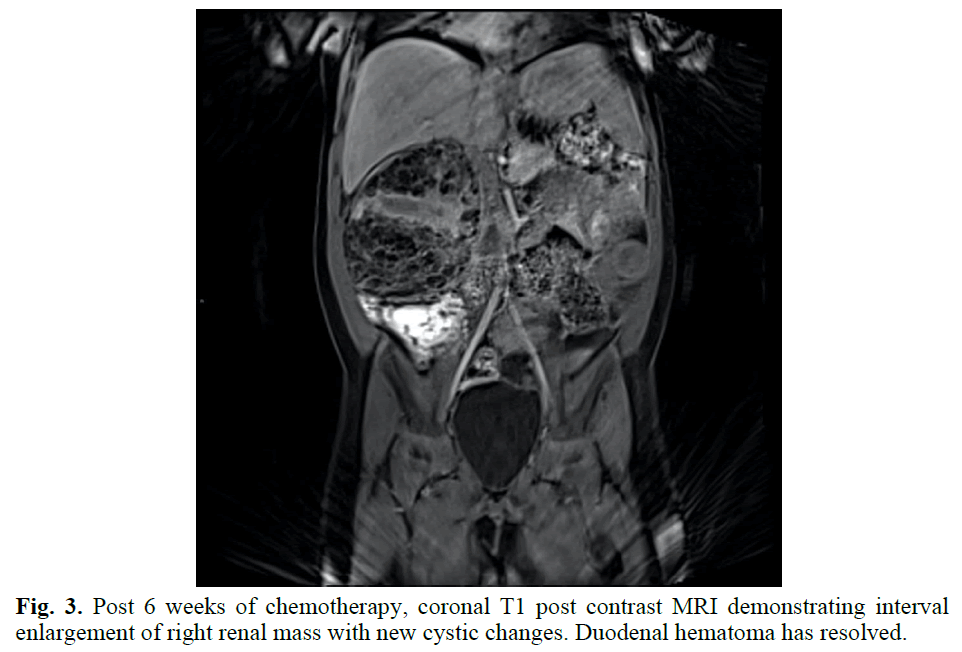
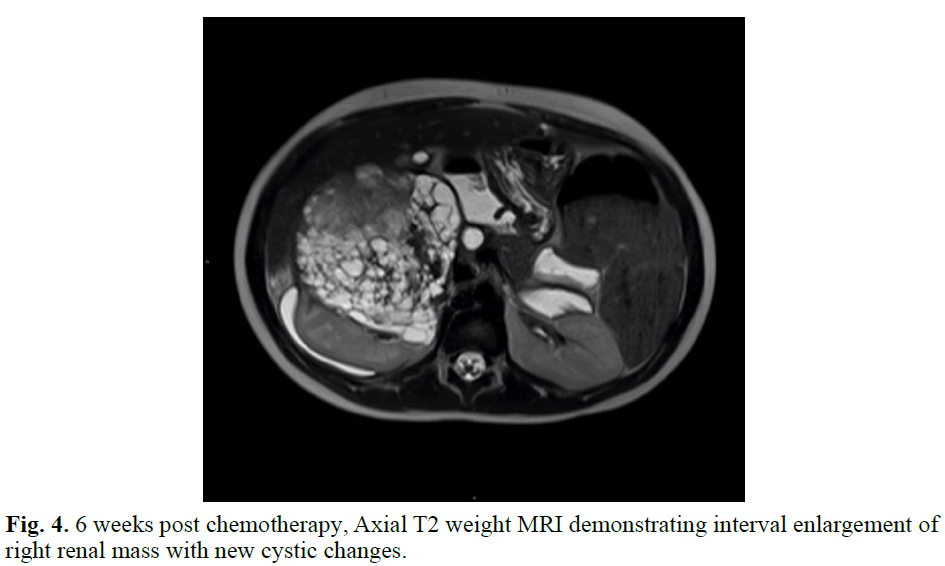
The patient was taken to the Operating Room (OR) on hospital day 3 to further evaluate the complex right renal mass with no intravascular tumor thrombus identified on pre-operative imaging, although a large intraluminal duodenal hematoma was present. Upon direct visualization and manipulation, it was deemed that to attempt to fully remove the right renal mass there would be significant risk of catastrophic caval injury as the mass was densely adherent to the IVC. The large intra- luminal duodenal-jejunal hematoma was visualized with resultant friable tissue on manipulation. Biopsies of the mass were obtained and sent to pathology. The pathology slides were sent out for second opinion, with findings of undifferentiated cells most consistent with Wilms Tumor given location of the right renal mass. The WT1 stain was negative, as can occur in up to 15% of WTs. There was no anaplasia. Results supported a diagnosis of WT with favorable histology (Figures 3 and 4).
Fig. 3. Post 6 weeks of chemotherapy, coronal T1 post contrast MRI demonstrating interval enlargement of right renal mass with new cystic changes. Duodenal hematoma has resolved.
Fig. 4. 6 weeks post chemotherapy, Axial T2 weight MRI demonstrating interval enlargement of right renal mass with new cystic changes.
Therapeutic intervention
The patient was treated with amlodipine for hypertension, acetaminophen and narcotics for pain control, gastritis prophylaxis with pantoprazole, ondansetron for nausea, and she was kept NPO. She received one unit pRBCs for anemia on HOD #2 prior to going to Operating Room (OR) for exploration on HOD #3. Under general anesthesia, an arterial line and epidural catheter were placed along with a 10-French Foley and prophylactic antibiotics with Cefazolin. A right upper quadrant transverse incision was made, with care to avoid injury to underlying tissues. In the abdominal cavity, intraperitoneal blood was evacuated which allowed the mass to be visualized. The proximal duodenum was completely flattened and adherent to the medial aspect of the tumor. The transverse colon was also adherent to the tumor. Firstly, the right colon was mobilized followed by the transverse colon by removing it from anterior aspect of tumor, avoiding injury to the colonic mesentery in the process. After the anterior surface of the tumor was exposed, the surgeons entered the plane between the duodenum and tumor capsule where the very large anti mesenteric duodenal hematoma was encountered tracking posteriorly. The clot had dissected the serial wall from the first and second portions of the duodenum and extended to the first 5 cm of the jejunum. There was no clear etiology of the hematoma, no vascular injury, nor evidence of tumor invasion. There was no evidence of duodenal perforation. The right kidney was subsequently mobilized superiorly, inferiorly, anteriorly, laterally, and posteriorly. Medially, there was dense tissue overlying the kidney, along with duodenal inflammation. Medial mobilization was unable to be achieved due to concern of damaging renal hilar structures further. Rather than proceed with nephrectomy, biopsies were obtained, and neoadjuvant chemotherapy was given prior to repeat imaging to evaluate for resolution of the hematoma. A G-J tube was placed for both decompression of the abdomen, and to allow for enteral nutrition distal to the obstructing duodenal hematoma. An NG tube was also placed for further GI decompression.
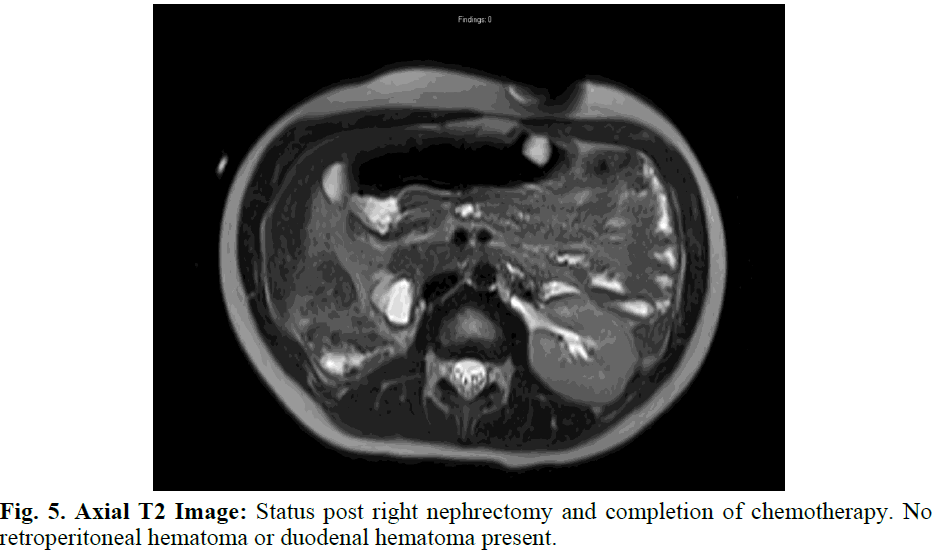
The patient received supportive care until pathology results returned on the renal mass, confirming Wilms Tumor with Favorable Histology. The patient subsequently completed 25 weeks of chemotherapy per AREN0533 DD-4A and continues routine follow up to evaluate for recurrent disease (Figure 5).
Fig. 5. Axial T2 Image: Status post right nephrectomy and completion of chemotherapy. No retroperitoneal hematoma or duodenal hematoma present.
Results and Discussion
Wilms Tumors are one of the more common pediatric solid tumors, accounting for 5% of pediatric tumors. This case represents a previously unreported presentation with an obstructing intra-luminal duodenal hematoma. The etiology of the hematoma is unclear at this time. Wilms Tumors and other masses have been known to present with hemorrhage and hematoma. There are reports in the literature of renal masses with resultant perirenal hemorrhage or subcapsular hemorrhage. Due to the presence of hemorrhage, if masses are not initially observed on imaging, they can frequently be misdiagnosed as trauma or related to splenomegaly. There are no reported cases of WT presenting with intraluminal duodenal hematoma.
Other examples of atypical initial presentations of WT include a mediastinal mass with lymphadenopathy and pulmonary nodules found on biopsy to be metastatic WT [4], and the renal isthmus of a horseshoe kidney with extension to inferior mesenteric vein, iliac vein, ureters, and IVC, aorta, and sacral promontory [5]. Renal neoplasms appear to be the most common cause of retroperitoneal hemorrhage caused by masses. There are rare cases reported of retroperitoneal sarcoma with hemorrhage, ruptured retroperitoneal lymph nodes with hemorrhage in a patient with metastatic mixed germ cell tumor. None of these have been noted to cause intraluminal duodenal hematoma.
Isolated duodenal hematomas are rare. There have been a few cases of duodenal hematoma after patients have undergone upper GI endoscopy. One case series focused on hematomas found after endoscopy was performed in patients with graft vs host disease [6]. Retroperitoneal hematomas have been reported to rarely cause duodenal obstruction, but they are typically due to mass effect of the hemorrhage, rather than intraluminal hematoma.
Traumatic duodenal hematomas are reported to most commonly occur in the second portion of the duodenum. Rarely, percutaneous drainage has been performed for acute bleeding. However, typically duodenal hematomas are managed nonoperatively [7-12].
Conclusion
For this patient, the cause of intraluminal duodenal hematoma is still unclear. Possibilities include microscopic intraluminal invasion that was not visible, unknown and unreported trauma that may have led to a GI bleed given the proximity and mass effect of the tumor, or other tumor associated hemorrhage. Intestinal decompression with G-J Tube and NG tube allowed the hematoma to be resorbed, and on return to the OR, dissection of the tumor from the surrounding structures was successful.
References
- Friedman AD. Wilms Tumor. Pediatr Rev. 2013;34(7):328-330.
[Crossref] [Google Scholar] [PubMed]
- Watanabe N, Omagari D, Yamada T, et al. Anaplastic sarcoma of the kidney: Case report and literature review. Pediatr Int. 2013;55(5):e129-132.
[Crossref] [Google Scholar] [PubMed]
- Lamalmi N, Rous L, Cherradi N, et al. Botryoid Wilms tumor extending into the duodenum. Arch Pediatr. 2010;17(12):1664-1666.
[Crossref] [Google Scholar] [PubMed]
- Diaconescu AC, Dougherty D, Ehrlich PF, et al. An unusual case of a Wilms Tumor presenting as a mediastinal mass. J Pediatr Surg Case Rep. 2022;87(1):102486.
- Fachin CG, Oliveria LD, Jamur CM, et al. Unusual Wilms tumors: Case series. J Pediatr Surg Case Rep. 2021;72(1):101971.
- Bergero G, Frangi D, Busoni V, et al. Duodenal Hematoma Post-Upper Digestive Endoscopy for Diagnosis of Graft Versus Host Disease in Pediatrics: About Two Cases. Arch Argent Pediatr. 2021;119(5):e513-517.
- Peterson ML, Abbas PI, Fallon SC, et al. Management of traumatic duodenal hematomas in children. J Surg Res. 2015;199(1):126-129.
[Crossref] [Google Scholar] [PubMed]
- Merali N, Singh G, Ghorpade A, et al. Idiopathic retroperitoneal haematoma causing duodenal obstruction: A case report and review of literature. Ann R Coll Surg Eng. 2020;102(8):e209-212.
[Crossref] [Google Scholar] [PubMed]
- Byerly D, Coley B, Ruymann F. Perirenal hemorrhage as first presentation of Wilms Tumor. Pediatr Radiol. 2006;36(7):714-717.
[Crossref] [Google Scholar] [PubMed]
- Rutigliano DN, Kayton ML, Steingerz P, et al. The use of preoperative chemotherapy in Wilms’ Tumor with contained retroperitoneal rupture. J Pediatr Surg. 2007;42(9):1595-1599.
[Crossref] [Google Scholar] [PubMed]
- Davidoff AM. Wilms Tumor. Adv Pediatr. 2012;59(1):247267.
[Crossref] [Google Scholar] [PubMed]
- Aldrink JH, Heaton TE, Dasgupta R, et al. Update on Wilms Tumor. J Pediatr Sur. 2019;54(3):390 -397.
[Crossref] [Google Scholar] [PubMed]
Author Info
Ethan Sandler1*, Helena Crowley2, Vijay Krishnan3, Hamad Aljutaili3, Maram Aljuaid3,4 and Regina Macatangay52Division of Pediatric Surgery, University of Maryland School of Medicine, Baltimore, Maryland, USA
3Division of Pediatric Radiology, University of Maryland School of Medicine, Baltimore, Maryland, USA
4Department of Radiology, King Saud University, Riyadh, Saudi Arabia
5Division of Hematology and Oncology, University of Maryland School of Medicine, Baltimore, Maryland, USA
Received: 07-Jan-2024, Manuscript No. PUCR-24-129106; , Pre QC No. PUCR-24-129106; Editor assigned: 11-Jan-2024, Pre QC No. PUCR-24-129106; Reviewed: 25-Jan-2024, QC No. PUCR-24-129106; Revised: 01-Feb-2024, Manuscript No. PUCR-24-129106; Published: 08-Feb-2024, DOI: 10.14534/j-pucr.20222675631
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.